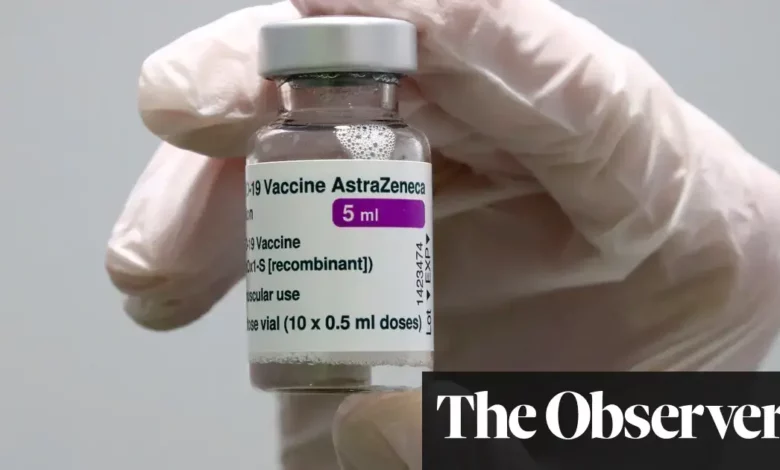
The partnership between AstraZeneca and Oxford University has been controversial. Researchers found an increased incidence of vaccine-induced immune thrombocytopenia and thrombosis (VITT) among AstraZeneca Covid-19 vaccine recipients.
This vaccine, called Covishield in India and Vaxzevria in Europe, has been associated with a rare blood clotting disorder that is sometimes fatal. The aetiology of VITT was discovered during the COVID-19 pandemic and revolves around a dangerous blood autoantibody targeting a protein called platelet factor 4 (PF4).
The recent research results from Flinders University in Australia and international experts show that both infections by adenovirus connected to VITT and classic adenoviral vector VITT had the same molecular fingerprint.
These results not only indicate genetic risk factors and pathways for antibody production but also have significance paradoxically for vaccine safety and development. As a result of this admission and withdrawal of marketing authorization in different global markets, AstraZeneca has acknowledged this rare side effect in a legal document.



